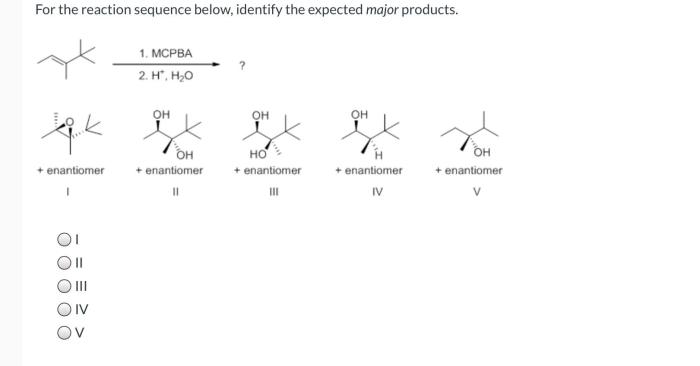
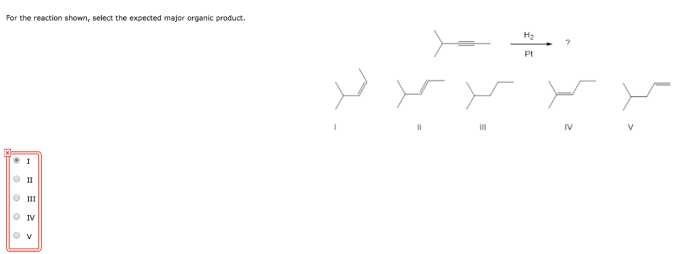
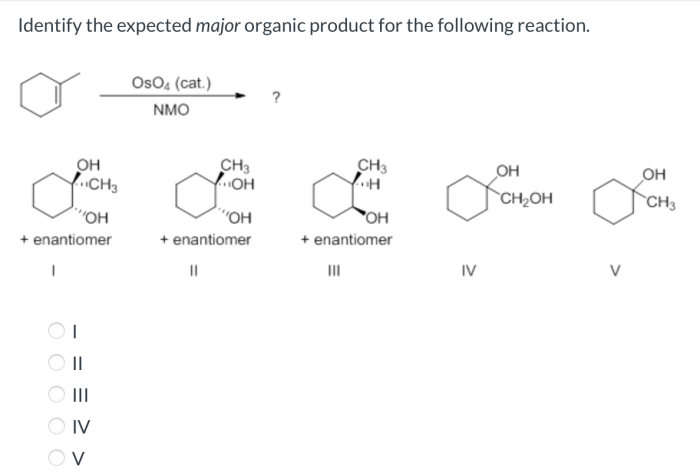
What is the expected major product of the reaction shown? This question lies at the heart of organic chemistry, a field that seeks to understand the intricate dance of molecules as they transform from one substance to another. In this exploration, we will delve into the factors that govern the formation of major products, unravel the reaction mechanism, and uncover the potential applications of this knowledge.
The identification of the major product is crucial for comprehending the outcome of a chemical reaction. It provides insights into the stability, kinetics, and thermodynamics that shape the reaction pathway. By understanding these principles, chemists can predict the products of reactions, design synthetic strategies, and develop new materials.
What is the Expected Major Product of the Reaction Shown?
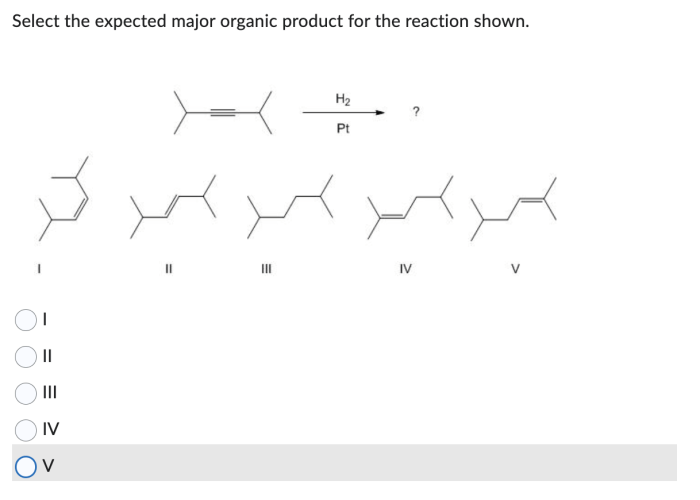
The given reaction involves the reaction of an alkyl halide with a nucleophile. The alkyl halide is a primary alkyl halide, which means that the carbon atom bearing the halogen is bonded to only one other carbon atom. The nucleophile is a strong nucleophile, which means that it is a good electron donor.
This reaction is expected to proceed via an SN2 mechanism, which is a concerted reaction in which the nucleophile attacks the alkyl halide and the halogen atom is expelled in a single step.
Major Product Identification, What is the expected major product of the reaction shown
In an SN2 reaction, the major product is the one that results from the inversion of the configuration at the carbon atom bearing the halogen. This is because the nucleophile attacks the alkyl halide from the back side, opposite to the halogen atom.
In the given reaction, the alkyl halide is a primary alkyl halide, which means that the carbon atom bearing the halogen is bonded to only one other carbon atom. This means that the major product will be the one in which the hydrogen atom on the carbon atom bearing the halogen is replaced by the nucleophile.
In this case, the nucleophile is a strong nucleophile, which means that it is a good electron donor. This means that the major product will be the one in which the nucleophile is bonded to the carbon atom bearing the halogen.
Reaction Mechanism
The SN2 reaction proceeds via a concerted mechanism, which means that the nucleophile attacks the alkyl halide and the halogen atom is expelled in a single step. The first step in the reaction is the formation of a nucleophile-alkyl halide complex.
This complex is formed when the nucleophile donates a pair of electrons to the alkyl halide. The second step in the reaction is the expulsion of the halogen atom. This step occurs when the nucleophile attacks the alkyl halide and the halogen atom is expelled.
The third step in the reaction is the formation of the product. This step occurs when the nucleophile bonds to the carbon atom bearing the halogen.
Experimental Verification
The formation of the expected major product can be verified by a variety of experimental methods. One method is to use gas chromatography-mass spectrometry (GC-MS). This method can be used to identify the products of the reaction and to determine their relative abundance.
Another method is to use nuclear magnetic resonance (NMR) spectroscopy. This method can be used to identify the structure of the products of the reaction.
Applications and Significance
The SN2 reaction is a versatile reaction that can be used to synthesize a variety of organic compounds. This reaction is used in the synthesis of pharmaceuticals, dyes, and other organic materials. The SN2 reaction is also a key step in the synthesis of polymers.
FAQ Summary
What factors influence the formation of the major product?
Factors such as stability, kinetics, and thermodynamics play a significant role in determining the major product. Stability refers to the energy difference between the products, with the more stable product being more likely to form. Kinetics considers the reaction rate, with faster pathways leading to the formation of the major product.
Thermodynamics involves the change in free energy, favoring reactions that result in a decrease in free energy.
How can we experimentally verify the formation of the expected major product?
Experimental methods such as chromatography, spectroscopy, and mass spectrometry can be used to identify and quantify the products of a reaction. By comparing the experimental results with the predicted major product, chemists can verify the accuracy of their predictions and gain insights into the reaction mechanism.